
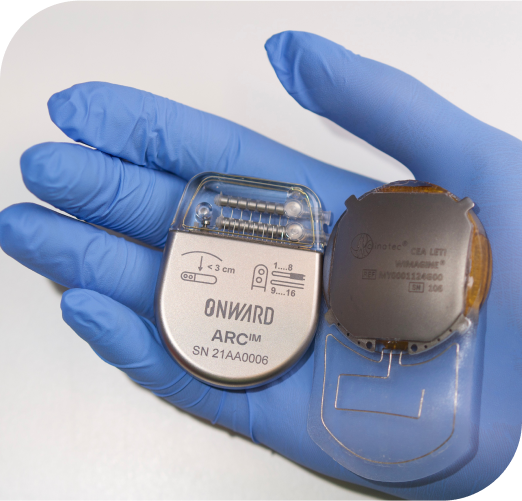
ARCIM is our investigational spinal cord stimulation system designed to address blood pressure instability and other symptoms in people with SCI.
Read the Evidence

The STIMO study demonstrated the ability of this therapy to enable long-paralyzed people to stand and walk again with little or no assistance.
We received FDA Breakthrough Device Designation for ARCIM in 2020 to restore leg motor function in people with spinal cord injury. Then in 2021, for the use of the system to normalize blood pressure and provide trunk stability, in 2022 for bladder control, and in 2023 for reduced spasticity.
Other potential indications we may explore include improved sexual function and bowel control, and Parkinson’s mobility and gait.
Join our Patient Registry if you are interested in our clinical trials
Discover the Potential Benefits
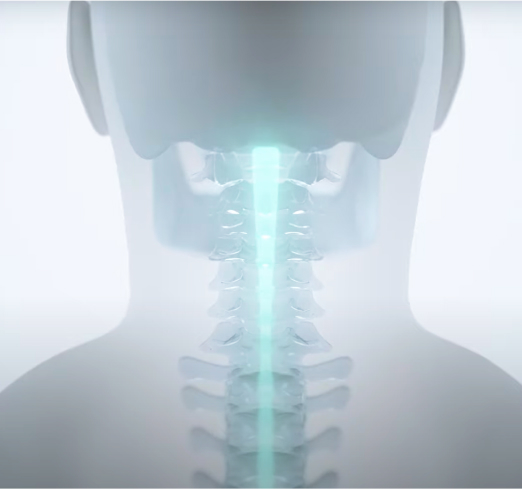
ARCIM utilizes ARC Therapy, which is targeted, programmed electrical stimulation of the spinal cord at the site of the injury to restore movement or a specific function.
The system consists of an implanted Neurostimulator (IPG) and an implanted epidural lead designed specifically for people with SCI.
Discover the Potential Benefits
ARCIM utilizes ARC Therapy, which is targeted, programmed electrical stimulation of the spinal cord at the site of the injury to restore movement or a specific function.
The system consists of an implanted Neurostimulator (IPG) and an implanted epidural lead designed specifically for people with SCI.
Blood pressure regulation
Upper and lower limb function
Bladder control

Improved Blood Pressure Regulation
ARCIM is the first neuroprosthetic system designed to reduce the symptoms related to Orthostatic and Postprandial Hypotension and Autonomic Dysreflexia (AD) in people living with chronic spinal cord injury (SCI) at lesion level C3-T6 AIS A-D.
ARCBCI
Learn about how we are pairing brain-computer interface (BCI) technology to enhance our ARCIM platform with more natural movement after paralysis.