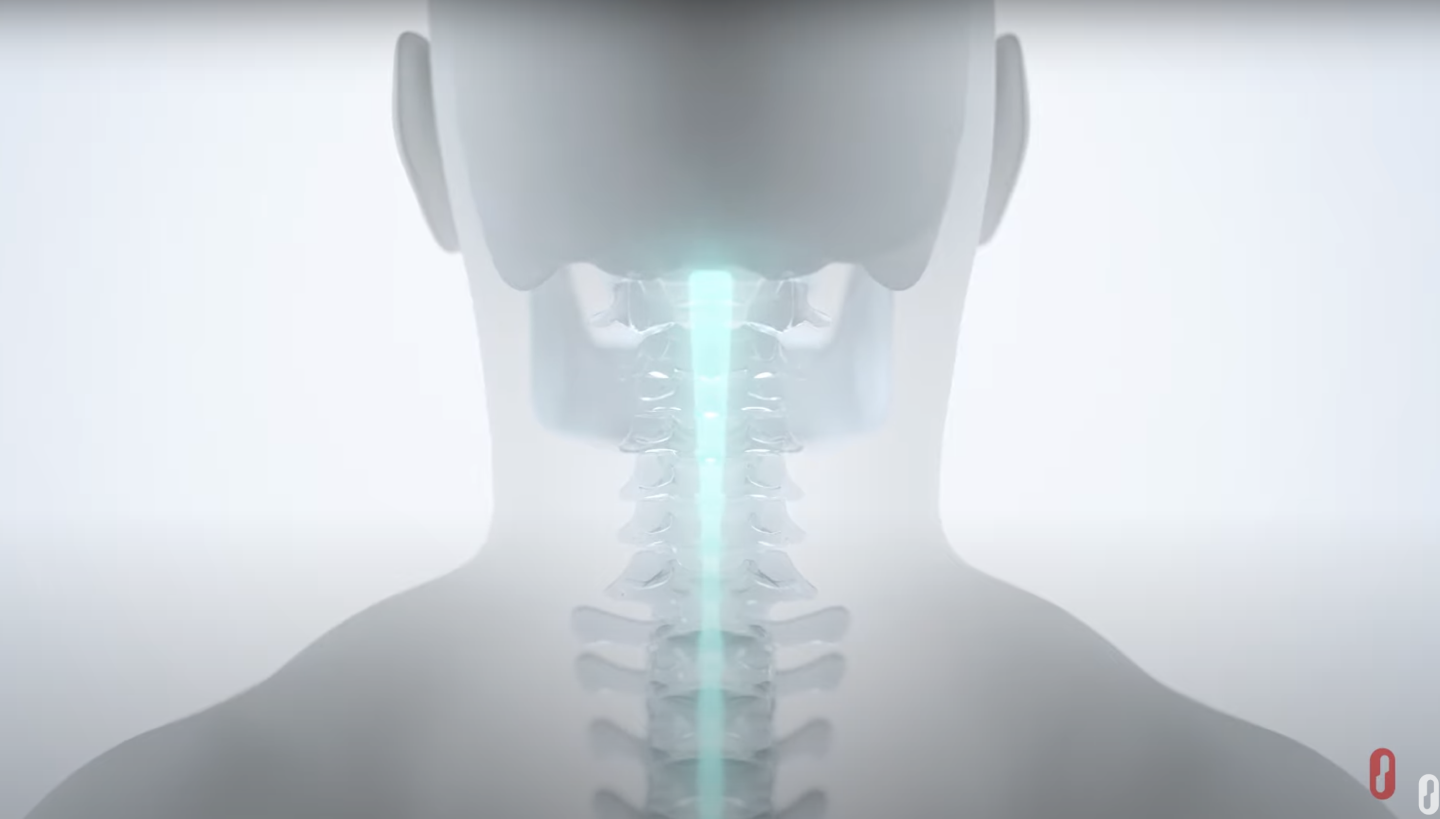
Lower Limb Movement
Discover our Therapy
Discover our Therapy
Implantable Platform
Implantable Platform
Read the Evidence

The STIMO study demonstrated the ability of this therapy to enable long-paralyzed people to stand and walk again with little or no assistance.
We received FDA Breakthrough Device Designation for ARCIM in 2020 to restore leg motor function in people with spinal cord injury. Then in 2021, for the use of the system to normalize blood pressure and provide trunk stability, in 2022 for bladder control, and in 2023 for reduced spasticity.
Other potential indications we may explore include improved sexual function and bowel control, and Parkinson’s mobility and gait.
ARCIM + BCI (brain-computer interface)
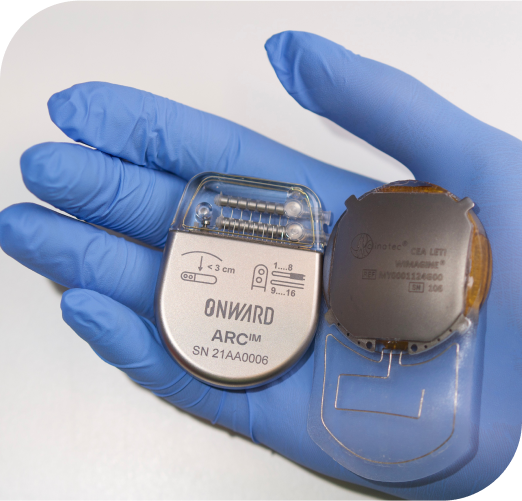
We are advancing brain-computer interface (BCI) technology to enhance our ARCIM platform with more natural movement after paralysis.
In partnership with EPFL and CEA-Clinatec, in 2021 we successfully implanted an investigational BCI in a human to augment our ARCIM Therapy with thought-driven movement of the legs after paralysis. The approach was published in Nature in May 2023.
With a European Innovation Council grant to study restoration of thought-driven arm and hand movement after SCI, an additional human was implanted with ARCIM Therapy + BCI in 2023. Three more people are scheduled to participate in the study in 2024.