Discover our Therapy
Discover our Therapy

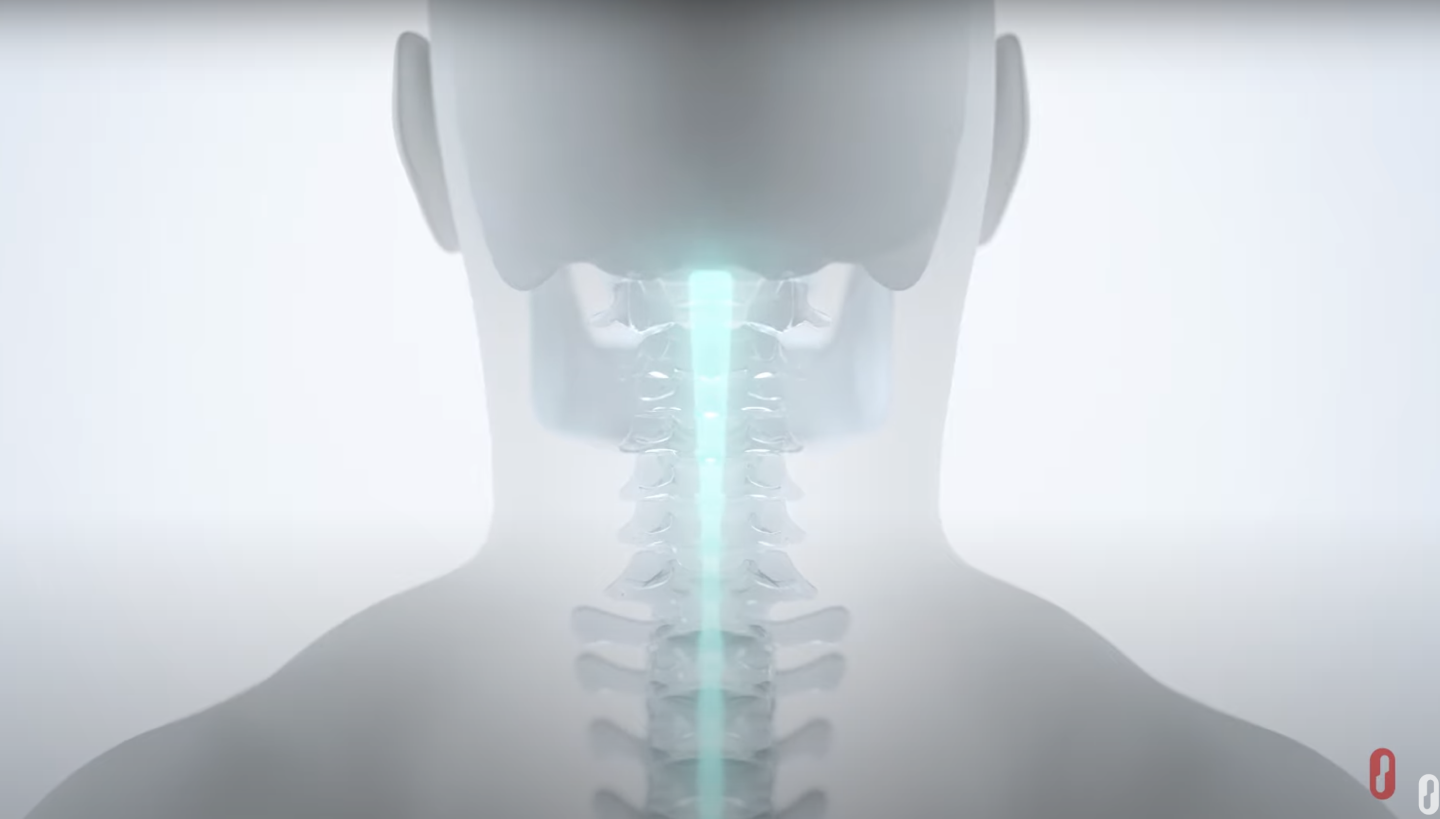
ARC Therapy is targeted stimulation of the spinal cord designed to restore movement and other critical functions after spinal cord injury, such as blood pressure stability and bladder control.
These benefits have been observed in published pre-clinical and human feasibility studies.
Clinical results may vary.
Watch how it works
-

ARC Therapy is targeted stimulation of the spinal cord designed to restore movement and other critical functions after spinal cord injury, such as blood pressure stability and bladder control.
These benefits have been observed in published pre-clinical and human feasibility studies.
Clinical results may vary.
Watch how it works
Implantable Platforms
Implantable Platforms

Investigational, implantable neurostimulation platform to minimally invasively deliver targeted ARCIM Therapy to the spinal cord.
The US FDA awarded the ARCIM platform Breakthrough Device Designations for restoration of leg motor function, blood pressure regulation, trunk stabilization, bladder control, and alleviation of spasticity in people with spinal cord injury.
We are also advancing brain-computer interface (BCI) technology to restore thought-driven movement after SCI when used with our ARCIM platform.
Learn more
-

Investigational, implantable neurostimulation platform to minimally invasively deliver targeted ARCIM Therapy to the spinal cord.
The US FDA awarded the ARCIM platform Breakthrough Device Designations for restoration of leg motor function, blood pressure regulation, trunk stabilization, bladder control, and alleviation of spasticity in people with spinal cord injury.
We are also advancing brain-computer interface (BCI) technology to restore thought-driven movement after SCI when used with our ARCIM platform.
Learn more

The ARCBCI System creates the ONWARD DigitalBridge™, reconnecting communication between the brain and the body to restore thought-driven movement after paralysis.
The ARCBCI System has been awarded US FDA Breakthrough Device Designation, the 10th such award for an ONWARD Medical technology or therapy. ONWARD is also one of the first neurotechnology companies invited to join the FDA’s Total Product Life Cycle Advisory (TAP) Program, providing early and frequent strategic engagement with the FDA and other stakeholders.
The ARCBCI System uses artificial intelligence (AI) to decode a person’s brain signals and translate their movement intentions into instructions for our ARCIM System. The ARCIM System then delivers precise spinal cord stimulation to restore thought-driven movement after paralysis.
-

The ARCBCI System creates the ONWARD DigitalBridge™, reconnecting communication between the brain and the body to restore thought-driven movement after paralysis.
The ARCBCI System has been awarded US FDA Breakthrough Device Designation, the 10th such award for an ONWARD Medical technology or therapy. ONWARD is also one of the first neurotechnology companies invited to join the FDA’s Total Product Life Cycle Advisory (TAP) Program, providing early and frequent strategic engagement with the FDA and other stakeholders.
The ARCBCI System uses artificial intelligence (AI) to decode a person’s brain signals and translate their movement intentions into instructions for our ARCIM System. The ARCIM System then delivers precise spinal cord stimulation to restore thought-driven movement after paralysis.
As part of a research consortium, in 2021 we successfully implanted the first investigational BCI in a human to enable thought-driven movement of the legs after paralysis. The results were published in Nature in May 2023.
In 2024, we secured exclusive rights to the WIMAGINE® BCI platform. WIMAGINE is a premier BCI technology with more than seven years of human safety data. The platform was originally developed by the prestigious French biomedical research institute, CEA-Clinatec. ONWARD now has the exclusive rights to develop and commercialize WIMAGINE as part of our ARCBCI System.
Through mid-2025, we have successfully implanted five humans with ARCBCI Therapy to facilitate mobility (walking) and upper extremity movement.
We expect to advance research and technology development of the ARCBCI System in the coming years, supported by grants from the Christopher & Dana Reeve Foundation and European Innovation Council.
Learn more
-
As part of a research consortium, in 2021 we successfully implanted the first investigational BCI in a human to enable thought-driven movement of the legs after paralysis. The results were published in Nature in May 2023.
In 2024, we secured exclusive rights to the WIMAGINE® BCI platform. WIMAGINE is a premier BCI technology with more than seven years of human safety data. The platform was originally developed by the prestigious French biomedical research institute, CEA-Clinatec. ONWARD now has the exclusive rights to develop and commercialize WIMAGINE as part of our ARCBCI System.
Through mid-2025, we have successfully implanted five humans with ARCBCI Therapy to facilitate mobility (walking) and upper extremity movement.
We expect to advance research and technology development of the ARCBCI System in the coming years, supported by grants from the Christopher & Dana Reeve Foundation and European Innovation Council.
Learn more
Please complete this form to
contact us or receive updates
The ONWARD® Medical ARCEX® System is cleared for use only in the United States. ONWARD ARCIM® and ARCBCI™, alone or in combination with a brain-computer interface (BCI), are investigational and not available for commercial use.